- Record: found
- Abstract: found
- Article: found
High-dose nimotuzumab improves the survival rate of esophageal cancer patients who underwent radiotherapy
Abstract
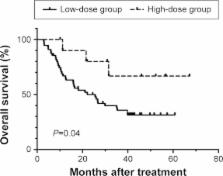
Nimotuzumab (h-R3) is a humanized monoclonal antibody that is safe to use against epidermal growth factor receptor (EGFR). However, the available information is insufficient about the dose effect of monoclonal antibody against epidermal growth factor receptor for the treatment of esophageal squamous cell carcinoma (ESCC). We retrospectively recruited 66 patients with ESCC who were treated with h-R3 and chemoradiotherapy/radiotherapy. Patients who received more than 1,200 mg of h-R3 were classified as the high-dose group, and the remaining patients were classified as the low-dose group. The endpoint for efficacy was the overall survival. Differences in survival between the groups were analyzed using the log-rank test. The Cox proportional hazards model was used in multivariate analysis to identify independent prognostic factors. The low-dose and high-dose groups comprised 55 and eleven patients, respectively. The median follow-up time in the final analysis was 46 months. The high-dose group showed no increased incidence of toxicities compared to the low-dose group. The 1-, 2-, and 5-year overall survival rates in the low-dose and high-dose groups were 66.9%, 50.0%, 31.5% and 90.0%, 80.0%, 66.7%, respectively ( P=0.04). Multivariate analyses showed that the high-dose group had better survival than the low-dose group (hazard ratio 0.28, 95% confidence interval 0.09–0.94, P=0.039). Taken together, high-dose h-R3 showed limited toxicity and improved survival in patients with ESCC.
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial.
- Record: found
- Abstract: found
- Article: not found
EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus.
- Record: found
- Abstract: found
- Article: not found