- Record: found
- Abstract: found
- Article: found
Naringenin modulates the NO-cGMP-PKG signaling pathway by binding to AKT to enhance osteogenic differentiation in hPDLSCs
Read this article at
Abstract
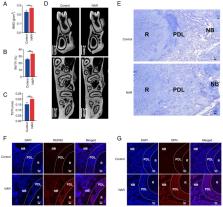
Naringenin (NAR) is a prominent flavanone that has been recognized for its capacity to promote the osteogenic differentiation of human periodontal ligament stem cells (hPDLSCs). The present study aimed to explore how NAR promotes the osteogenic differentiation of hPDLSCs and to assess its efficacy in repairing alveolar bone defects. For this purpose, a protein-protein interaction network of NAR action was established by mRNA sequencing and network pharmacological analysis. Gene and protein expression levels were evaluated by reverse transcription-quantitative and western blotting. Alizarin red and alkaline phosphatase staining were also employed to observe the osteogenic capacity of hPDLSCs, and immunofluorescence was used to examine the co-localization of NAR molecular probes and AKT in cells. The repair of mandibular defects was assessed by micro-computed tomography (micro-CT), Masson staining and immunofluorescence. Additionally, computer simulation docking software was utilized to determine the binding affinity of NAR to the target protein, AKT. The results demonstrated that activation of the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) signaling pathway could promote the osteogenic differentiation of hPDLSCs. Inhibition of AKT, endothelial nitric oxide synthase and soluble guanylate cyclase individually attenuated the ability of NAR to promote the osteogenic differentiation of hPDLSCs. Micro-CT and Masson staining revealed that the NAR gavage group exhibited more new bone formation at the defect site. Immunofluorescence assays confirmed the upregulated expression of Runt-related transcription factor 2 and osteopontin in the NAR gavage group. In conclusion, the results of the present study suggested that NAR promotes the osteogenic differentiation of hPDLSCs by activating the NO-cGMP-PKG signaling pathway through its binding to AKT.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: found
- Article: not found
AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility.
- Record: found
- Abstract: found
- Article: not found