- Record: found
- Abstract: found
- Article: found
Patient Expectations of Assigned Treatments Impact Strength of Randomised Control Trials
Read this article at
Abstract
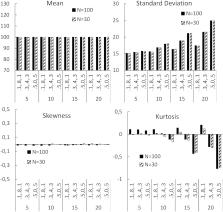
Patient engagement with treatments potentially poses problems for interpreting the results and meaning of Randomised Control Trials (RCTs). If patients are assigned to treatments that do, or do not, match their expectations, and this impacts their motivation to engage with that treatment, it will affect the distribution of outcomes. In turn, this will impact the obtained power and error rates of RCTs. Simple Monto Carlo simulations demonstrate that these patient variables affect sample variance, and sample kurtosis. These effects reduce the power of RCTs, and may lead to false negatives, even when the randomisation process works, and equally distributes those with positive and negative views about a treatment to a trial arm.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables.
- Record: found
- Abstract: found
- Article: not found
Assessing the quality of reports of randomized clinical trials: is blinding necessary?
- Record: found
- Abstract: not found
- Article: not found