- Record: found
- Abstract: found
- Article: found
Cancer cell‐derived exosomal miR‐20a‐5p inhibits CD8 + T‐cell function and confers anti‐programmed cell death 1 therapy resistance in triple‐negative breast cancer
Read this article at
Abstract
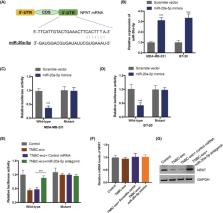
Circulating miRNAs (cirmiRNAs) can be packaged into the exosomes, participating in intercellular communication, which affects the malignant progression and therapy resistance of triple‐negative breast cancer (TNBC). Currently, immune checkpoint inhibitors that regulate T‐cell function, especially antibodies against programmed cell death 1 (PD‐1) or its ligand PD‐L1, are emerging as new promising therapy for TNBC patients. However, only very limited patients showed complete or partial response to anti‐PD‐1 treatment. Dysfunction of CD8 + T cells is one of the key reasons for the immune escape of TNBC. The regulation of exosome‐derived cirmiRNAs on CD8 + T cells in TNBC deserves more investigation. Here, the cirmiR‐20a‐5p level was significantly upregulated in the plasma of TNBC patients and culture supernatant of TNBC cells. High abundance of cirmiR‐20a‐5p was correlated with a worse prognosis of TNBC. cirmiR‐20a‐5p was secreted in the form of exosomes by TNBC cells. Exosomal cirmiR‐20a‐5p was internalized into CD8 + T cells and resulted into the dysfunction of CD8 + T. A mechanism study uncovered that cirmiR‐20a‐5p targeted the nuclear protein ataxia‐telangiectasia (NPAT) and decreased NPAT expression in CD8 + T cells. An in vivo xenograft mouse model showed that cirmiR‐20a‐5p conferred TNBC to anti‐PD‐1 treatment resistance. Collectively, these findings indicated that cirmiR‐20a‐5p released by TNBC cells via exosome promotes cancer cell growth and leads to the immunosuppression by inducing CD8 + T cell dysfunction. This study suggests that targeting cirmiR‐20a‐5p might be a novel strategy for overcoming the resistance of TNBC to anti‐PD‐1 immunotherapy.
Abstract
Exosomal cirmiR‐20a‐5p was upregulated in triple‐negative breast cancer (TNBC) and correlated with the poor prognosis of TNBC patients. cirmiR‐20a‐5p released via exosomes by TNBC cells was uptaken by CD8 + T cells and led to the dysfunction of CD8 + T cells by targeting nuclear protein ataxia‐telangiectasia. Increased cirmiR‐20a‐5p enhanced the resistance of TNBC to anti‐PD‐1 therapy. These results suggest that targeting cirmiR‐20a‐5p may be a novel strategy for improving the immunotherapy efficacy of TNBC patients.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
The biology, function, and biomedical applications of exosomes
- Record: found
- Abstract: found
- Article: not found
Exosomal PD-L1 Contributes to Immunosuppression and is Associated with anti-PD-1 Response
- Record: found
- Abstract: found
- Article: found
