- Record: found
- Abstract: found
- Article: found
Stabilization of Polyoxometalate Charge Carriers via Redox‐Driven Nanoconfinement in Single‐Walled Carbon Nanotubes

Read this article at
Abstract
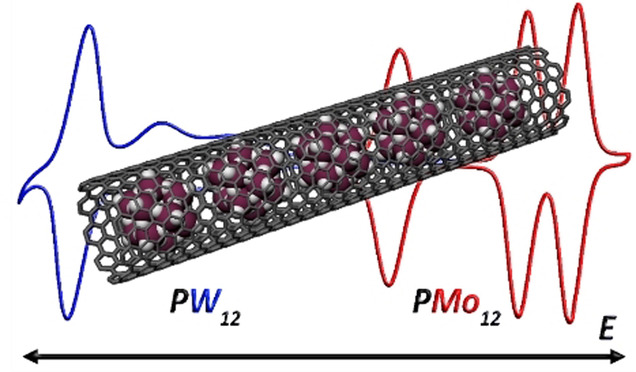
We describe the preparation of hybrid redox materials based on polyoxomolybdates encapsulated within single‐walled carbon nanotubes (SWNTs). Polyoxomolybdates readily oxidize SWNTs under ambient conditions in solution, and here we study their charge‐transfer interactions with SWNTs to provide detailed mechanistic insights into the redox‐driven encapsulation of these and similar nanoclusters. We are able to correlate the relative redox potentials of the encapsulated clusters with the level of SWNT oxidation in the resultant hybrid materials and use this to show that precise redox tuning is a necessary requirement for successful encapsulation. The host–guest redox materials described here exhibit exceptional electrochemical stability, retaining up to 86 % of their charge capacity over 1000 oxidation/reduction cycles, despite the typical lability and solution‐phase electrochemical instability of the polyoxomolybdates we have explored. Our findings illustrate the broad applicability of the redox‐driven encapsulation approach to the design and fabrication of tunable, highly conductive, ultra‐stable nanoconfined energy materials.
Abstract
The wide applicability of the redox‐driven encapsulation of polyoxometalates (POMs) within single‐walled carbon nanotubes is shown, demonstrating that the level of carbon oxidation and the efficacy of the filling are determined by the frontier orbital energies of the POM. Nanoencapsulation is found to confer significant stability to polyoxomolybdates despite their inherent lability.
Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found