- Record: found
- Abstract: found
- Article: not found
Insight into the role of clathrin‐mediated endocytosis inhibitors in SARS‐CoV‐2 infection
Read this article at
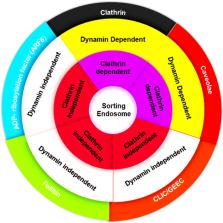
Abstract
Emergence of SARS‐CoV‐2 variants warrants sustainable efforts to upgrade both the diagnostic and therapeutic protocols. Understanding the details of cellular and molecular basis of the virus–host cell interaction is essential for developing variant‐independent therapeutic options. The internalization of SARS‐CoV‐2, into lung epithelial cells, is mediated by endocytosis, especially clathrin‐mediated endocytosis (CME). Although vaccination is the gold standard strategy against viral infection, selective inhibition of endocytic proteins, complexes, and associated adaptor proteins may present a variant‐independent therapeutic strategy. Although clathrin and/or dynamins are the most important proteins involved in CME, other endocytic mechanisms are clathrin and/or dynamin independent and rely on other proteins. Moreover, endocytosis implicates some subcellular structures, like plasma membrane, actin and lysosomes. Also, physiological conditions, such as pH and ion concentrations, represent an additional factor that mediates these events. Accordingly, endocytosis related proteins are potential targets for small molecules that inhibit endocytosis‐mediated viral entry. This review summarizes the potential of using small molecules, targeting key proteins, participating in clathrin‐dependent and ‐independent endocytosis, as variant‐independent antiviral drugs against SARS‐CoV‐2 infection. The review takes two approaches. The first outlines the potential role of endocytic inhibitors in preventing endocytosis‐mediated viral entry and its mechanism of action, whereas in the second computational analysis was implemented to investigate the selectivity of common inhibitors against endocytic proteins in SARS‐CoV‐2 endocytosis. The analysis revealed that remdesivir, methyl‐β‐cyclodextrin, rottlerin, and Bis‐T can effectively inhibit clathrin, HMG‐CoA reductase, actin, and dynamin I GTPase and are more potent in inhibiting SARS‐CoV‐2 than chloroquine. CME inhibitors for SARS‐CoV‐2 infection remain understudied.
Related collections
Most cited references240
- Record: found
- Abstract: found
- Article: not found
Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses
- Record: found
- Abstract: found
- Article: not found
