- Record: found
- Abstract: found
- Article: found
Surface α-Enolase Promotes Extracellular Matrix Degradation and Tumor Metastasis and Represents a New Therapeutic Target
Read this article at
Abstract
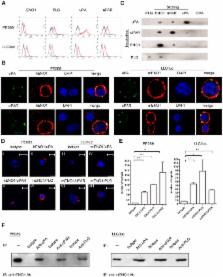
In previous research, we found α-enolase to be inversely correlated with progression-free and overall survival in lung cancer patients and detected α-enolase on the surface of lung cancer cells. Based on these findings, we hypothesized that surface α-enolase has a significant role in cancer metastasis and tested this hypothesis in the current study. We found that α-enolase was co-immunoprecipitated with urokinase-type plasminogen activator, urokinase-type plasminogen activator receptor, and plasminogen in lung cancer cells and interacted with these proteins in a cell-free dot blotting assay, which can be interrupted by α-enolase-specific antibody. α-Enolase in lung cancer cells co-localized with these proteins and was present at the site of pericellular degradation of extracellular matrix components. Treatment with antibody against α-enolase in vitro suppressed cell-associated plasminogen and matrix metalloproteinase activation, collagen and gelatin degradation, and cell invasion. Examination of the effect of treatment with shRNA plasmids revealed that down regulation of α-enolase decreases extracellular matrix degradation by and the invasion capacity of lung cancer cells. Adoptive transfer of α-enolase-specific antibody to mice resulted in accumulation of antibody in subcutaneous tumor and inhibited the formation of tumor metastasis in lung and bone. This study demonstrated that surface α-enolase promotes extracellular matrix degradation and invasion of cancer cells and that targeting surface α-enolase is a promising approach to suppress tumor metastasis.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Multifunctional alpha-enolase: its role in diseases.
- Record: found
- Abstract: found
- Article: not found
Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis.
- Record: found
- Abstract: found
- Article: not found