- Record: found
- Abstract: found
- Article: found
Up-regulation of multiple proteins and biological processes during maxillary expansion in rats
Read this article at
Abstract
Background
Maxillary expansion (ME) is a common practice in orthodontics that aims to increase the constricted maxillary arch width. Relapse often occurs, however, and better treatment strategies are needed. In order to develop a more effective method, this study was designed to further examine the process of tissue remodeling during ME, to identify the changes in expression of several proteins of interest, and to clarify the molecular mechanism responsible for tissue remodeling.
Methods
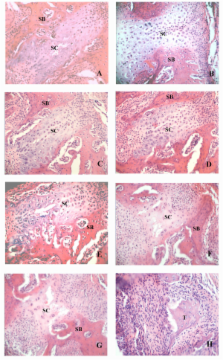
Male Wistar rats were randomly divided into control and ME groups. The rats were euthanized at various intervals over 11 days, and the dissected palates were prepared for histological examination. The structure of the midpalatal sutures changed little during the first three days. Proteins from samples in the ground midpalatal tissues obtained on the third day were subjected to two-dimensional polyacrylamide gel electrophoresis (2-DE) and matrix assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis. Validation of protein expression was performed by Western blot analyses.
Results
From day 5, chondrocytes in the inner layer of suture cartilage and osteoblasts at the end of the suture cartilage began to proliferate, and the skeletal matrix increased later adjacent to the cartilage in the ME group. Comparative proteomic analysis showed increases in 22 protein spots present in the ME group. The changes in three proteins closely related to osteogenesis (parathyroid hormone, osteoprotegerin and vimentin) were confirmed by Western blotting.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure.
- Record: found
- Abstract: found
- Article: not found
ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1).
- Record: found
- Abstract: found
- Article: not found