- Record: found
- Abstract: found
- Article: found
TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells
Read this article at
Abstract
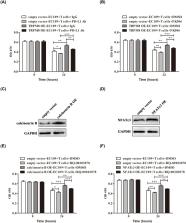
Esophageal cancer is seen with increasing incidence, but the underlying mechanism of esophageal cancer is still unknown. Transient receptor potential melastatin (TRPM) is non-selective cation channels. It has been verified that TRPM channels play crucial roles in development and progression of multiple tumors. Increasing studies have shown that TRPM8, a member of TRPM channels, promotes growth of tumors. However, it is still unclear whether TRPM8 has biological effect on esophageal cancer. In the current work, we found that TRPM8 was overexpressed in esophageal cancer samples and cell lines. Further investigation revealed that TRPM8 promoted proliferation of esophageal cancer cells. Next, the co-incubation assay including EC109 cells and CD8 + T cells revealed that TRPM8 overexpression and TRPM8 agonist reduced the cytotoxic effect of CD8 + T cell on esophageal cancer cells. Finally, we explored the mechanism and found that calcineurin-nuclear factor of activated T cells 3 (NFATc3) pathway contributed to the expression of programmed death ligand 1 (PD-L1) induced by TRPM8 overexpression and TRPM8 agonist, which might lead to immune evasion of esophageal cancer cells. These findings uncovered the crucial role of TRPM8 in the pathogenesis of esophageal cancer.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
A TRP channel that senses cold stimuli and menthol.
- Record: found
- Abstract: found
- Article: not found
Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma.
- Record: found
- Abstract: found
- Article: not found