- Record: found
- Abstract: found
- Article: found
The latest breakthrough on NLRP6 inflammasome
Read this article at
Abstract
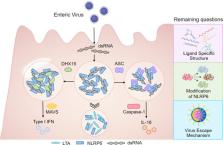
NLRP6, a Nod-like receptor family member, has been shown to affect intestinal homeostasis and microbial colonization through organizing a huge protein complex called inflammasome. NLRP6 inflammasome promotes the cleavage and secretion of inflammatory cytokines or the cleavage of pore-forming Gasdermin D to initiate the inflammatory cell death called pyroptosis, which plays important roles in responding to pathogen invasion. However, questions about the ligand(s) that trigger NLRP6 inflammasome activation, or the mechanisms that how a ligand triggers NLRP6 inflammasome assembly, are emerging. In this mini-review, we summarize the current understandings of ligand recognition of NLRP6, the role of liquid-liquid phase separation in NLRP6 inflammasome assembly, and potential links with human health and diseases.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Protein Phase Separation: A New Phase in Cell Biology
- Record: found
- Abstract: found
- Article: not found
Pathogen recognition and inflammatory signaling in innate immune defenses.
- Record: found
- Abstract: found
- Article: not found