- Record: found
- Abstract: found
- Article: found
Glioblastoma cells use an integrin- and CD44-mediated motor-clutch mode of migration in brain tissue
Read this article at
Summary
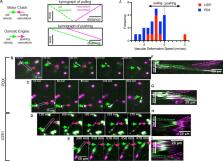
Glioblastoma (GBM) is an aggressive malignant brain tumor with 2-year survival rates of 6.7% [ 1], [ 2]. One key characteristic of the disease is the ability of glioblastoma cells to migrate rapidly and spread throughout healthy brain tissue[ 3], [ 4]. To develop treatments that effectively target cell migration, it is important to understand the fundamental mechanism driving cell migration in brain tissue. Here we utilized confocal imaging to measure traction dynamics and migration speeds of glioblastoma cells in mouse organotypic brain slices to identify the mode of cell migration. Through imaging cell-vasculature interactions and utilizing drugs, antibodies, and genetic modifications to target motors and clutches, we find that glioblastoma cell migration is most consistent with a motor-clutch mechanism to migrate through brain tissue ex vivo, and that both integrins and CD44, as well as myosin motors, play an important role in constituting the adhesive clutch.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Every step of the way: integrins in cancer progression and metastasis
- Record: found
- Abstract: found
- Article: not found
Integrins in cancer: biological implications and therapeutic opportunities.
- Record: found
- Abstract: found
- Article: not found