- Record: found
- Abstract: found
- Article: found
Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice

Read this article at
Abstract
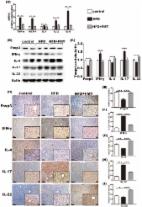
Alzheimer’s disease (AD) is the most common dementia in the elderly. Treatment for AD is still a difficult task in clinic. AD is associated with abnormal gut microbiota. However, little is known about the role of fecal microbiota transplantation (FMT) in AD. Here, we evaluated the efficacy of FMT for the treatment of AD. We used an APPswe/PS1dE9 transgenic (Tg) mouse model. Cognitive deficits, brain deposits of amyloid-β (Aβ) and phosphorylation of tau, synaptic plasticity as well as neuroinflammation were assessed. Gut microbiota and its metabolites short-chain fatty acids (SCFAs) were analyzed by 16S rRNA sequencing and 1H nuclear magnetic resonance (NMR). Our results showed that FMT treatment could improve cognitive deficits and reduce the brain deposition of amyloid-β (Aβ) in APPswe/PS1dE9 transgenic (Tg) mice. These improvements were accompanied by decreased phosphorylation of tau protein and the levels of Aβ40 and Aβ42. We observed an increases in synaptic plasticity in the Tg mice, showing that postsynaptic density protein 95 (PSD-95) and synapsin I expression were increased after FMT. We also observed the decrease of COX-2 and CD11b levels in Tg mice after FMT. We also found that FMT treatment reversed the changes of gut microbiota and SCFAs. Thus, FMT may be a potential therapeutic strategy for AD.
Related collections
Most cited references44

- Record: found
- Abstract: found
- Article: found
Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians
- Record: found
- Abstract: found
- Article: found
Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota
- Record: found
- Abstract: found
- Article: not found