- Record: found
- Abstract: found
- Article: found
Metabolic Features of Mouse and Human Retinas: Rods versus Cones, Macula versus Periphery, Retina versus RPE

Read this article at
Summary
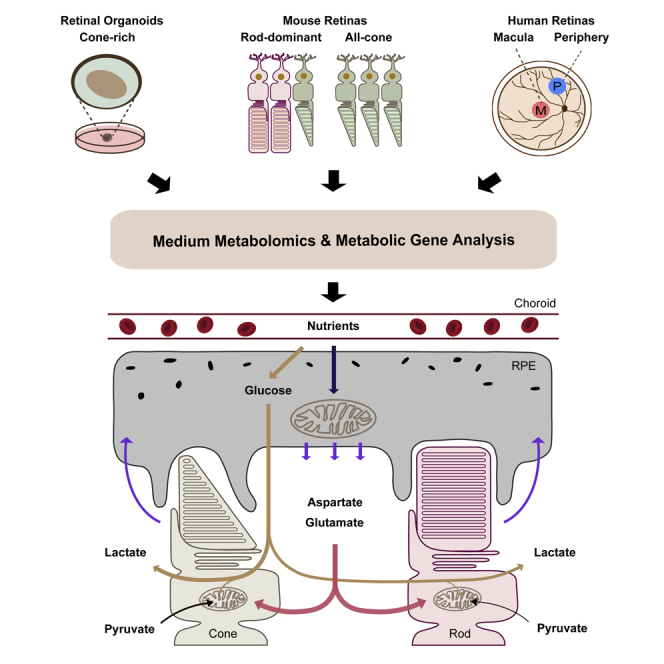
Photoreceptors, especially cones, which are enriched in the human macula, have high energy demands, making them vulnerable to metabolic stress. Metabolic dysfunction of photoreceptors and their supporting retinal pigment epithelium (RPE) is an important underlying cause of degenerative retinal diseases. However, how cones and the macula support their exorbitant metabolic demand and communicate with RPE is unclear. By profiling metabolite uptake and release and analyzing metabolic genes, we have found cone-rich retinas and human macula share specific metabolic features with upregulated pathways in pyruvate metabolism, mitochondrial TCA cycle, and lipid synthesis. Human neural retina and RPE have distinct but complementary metabolic features. Retinal metabolism centers on NADH production and neurotransmitter biosynthesis. The retina needs aspartate to sustain its aerobic glycolysis and mitochondrial metabolism. RPE metabolism is directed toward NADPH production and biosynthesis of acetyl-rich metabolites, serine, and others. RPE consumes multiple nutrients, including proline, to produce metabolites for the retina.
Graphical Abstract
Highlights
Abstract
Biochemistry; Sensory Neuroscience; Metabolomics
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Glucose feeds the TCA cycle via circulating lactate
- Record: found
- Abstract: found
- Article: not found
mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice.
- Record: found
- Abstract: found
- Article: not found