- Record: found
- Abstract: found
- Article: found
Non-specific Effect of Vaccines: Immediate Protection against Respiratory Syncytial Virus Infection by a Live Attenuated Influenza Vaccine
Read this article at
Abstract
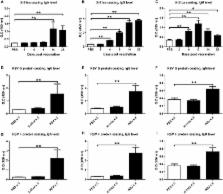
The non-specific effects (NSEs) of vaccines have been discussed for their potential long-term beneficial effects beyond direct protection against a specific pathogen. Cold-adapted, live attenuated influenza vaccine (CAIV) induces local innate immune responses that provide a broad range of antiviral immunity. Herein, we examined whether X-31ca, a donor virus for CAIVs, provides non-specific cross-protection against respiratory syncytial virus (RSV). The degree of RSV replication was significantly reduced when X-31ca was administered before RSV infection without any RSV-specific antibody responses. The vaccination induced an immediate release of cytokines and infiltration of leukocytes into the respiratory tract, moderating the immune perturbation caused by RSV infection. The potency of protection against RSV challenge was significantly reduced in TLR3 -/- TLR7 -/- mice, confirming that the TLR3/7 signaling pathways are necessary for the observed immediate and short-term protection. The results suggest that CAIVs provide short-term, non-specific protection against genetically unrelated respiratory pathogens. The additional benefits of CAIVs in mitigating acute respiratory infections for which vaccines are not yet available need to be assessed in future studies.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA.
- Record: found
- Abstract: found
- Article: not found
Innate immunity to influenza virus infection.
- Record: found
- Abstract: found
- Article: not found