- Record: found
- Abstract: found
- Article: found
Recent advances in the reconstruction of cranio-maxillofacial defects using computer-aided design/computer-aided manufacturing
Read this article at
Abstract
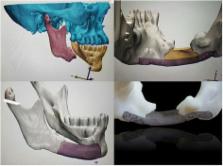
With the development of computer-aided design/computer-aided manufacturing (CAD/CAM) technology, it has been possible to reconstruct the cranio-maxillofacial defect with more accurate preoperative planning, precise patient-specific implants (PSIs), and shorter operation times. The manufacturing processes include subtractive manufacturing and additive manufacturing and should be selected in consideration of the material type, available technology, post-processing, accuracy, lead time, properties, and surface quality. Materials such as titanium, polyethylene, polyetheretherketone (PEEK), hydroxyapatite (HA), poly-DL-lactic acid (PDLLA), polylactide-co-glycolide acid (PLGA), and calcium phosphate are used. Design methods for the reconstruction of cranio-maxillofacial defects include the use of a pre-operative model printed with pre-operative data, printing a cutting guide or template after virtual surgery, a model after virtual surgery printed with reconstructed data using a mirror image, and manufacturing PSIs by directly obtaining PSI data after reconstruction using a mirror image. By selecting the appropriate design method, manufacturing process, and implant material according to the case, it is possible to obtain a more accurate surgical procedure, reduced operation time, the prevention of various complications that can occur using the traditional method, and predictive results compared to the traditional method.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: found
Selective laser sintering (SLS) 3D printing of medicines

- Record: found
- Abstract: found
- Article: found
Biocompatibility of Advanced Manufactured Titanium Implants—A Review

- Record: found
- Abstract: found
- Article: found