- Record: found
- Abstract: found
- Article: found
Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: from mechanistic study to catalyst design†
Read this article at
Abstract

Abstract
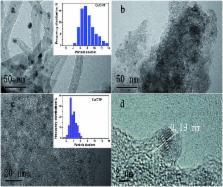
Development of non-noble metal catalysts with similar activity and stability to noble metals is of significant importance in the conversion and utilization of clean energy. The catalytic hydrolysis of ammonia borane (AB) to produce 3 equiv. of H 2, as an example of where noble metal catalysts significantly outperform their non-noble peers, serves as an excellent test site for the design and optimization of non-noble metal catalysts. Our kinetic isotopic effect measurements reveal, for the first time, that the kinetic key step of the hydrolysis is the activation of H 2O. Deducibly, a transition metal with an optimal electronic structure that bonds H 2O and –OH in intermediate strengths would favor the hydrolysis of AB. By employing a covalent triazine framework (CTF), a newly developed porous material capable of donating electrons through the lone pairs on N, the electron densities of nano-sized Co and Ni supported on CTF are markedly increased, as well as their catalytic activities. Specifically, Co/CTF exhibits a total turnover frequency of 42.3 mol H 2 mol Co –1 min –1 at room temperature, which is superior to all peer non-noble metal catalysts ever reported and even comparable to some noble metal catalysts.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
B-N compounds for chemical hydrogen storage.
- Record: found
- Abstract: found
- Article: not found
Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties
- Record: found
- Abstract: found
- Article: not found
The hydrogen issue.
Author and article information
Notes
†Electronic supplementary information (ESI) available: 11B NMR spectra, XRD patterns, results of BET and ICP, XPS spectra, TOF values and activation energies E a of the non-noble metal catalysts, time versus volume of H 2, catalytic activities and TEM images of 5% Co/CNT, 3% Co/CNT, 1% Co/CNT, the plot of hydrogen generation rate versus the concentration of Co and AB, kinetic isotope effect and TEM image of 5% Co/CTF-1 after reaction. See DOI: 10.1039/c6sc02456d