- Record: found
- Abstract: found
- Article: found
The Impact of the Cancer Microenvironment on Macrophage Phenotypes
Read this article at
Abstract
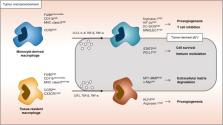
Within the tumor microenvironment, there is an intricate communication happening between tumor and stromal cells. This information exchange, in the form of cytokines, growth factors, extracellular vesicles, danger molecules, cell debris, and other factors, is capable of modulating the function of immune cells. The triggering of specific responses, including phenotypic alterations, can ultimately result in either immune surveillance or tumor cell survival. Macrophages are a well-studied cell lineage illustrating the different cellular phenotypes possible, depending on the tumor microenvironmental context. While our understanding of macrophage responses is well documented in vitro, surprisingly, little work has been done to confirm these observations in the cancer microenvironment. In fact, there are examples of opposing reactions of macrophages to cytokines in cell culture and in vivo tumor settings. Additionally, it seems that different macrophage lineages, for example tissue-resident and monocyte-derived macrophages, respond differently to cytokines and other cancer-derived signals. In this review article, we will describe and discuss the diverging reports on how cancer cells influence monocyte-derived and tissue-resident macrophage traits in vivo.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization.
- Record: found
- Abstract: not found
- Article: not found
Secretory products of macrophages.
- Record: found
- Abstract: found
- Article: not found