- Record: found
- Abstract: found
- Article: found
A Vimentin-Targeting Oral Compound with Host-Directed Antiviral and Anti-Inflammatory Actions Addresses Multiple Features of COVID-19 and Related Diseases
Read this article at
ABSTRACT
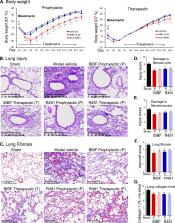
Damage in COVID-19 results from both the SARS-CoV-2 virus and its triggered overactive host immune responses. Therapeutic agents that focus solely on reducing viral load or hyperinflammation fail to provide satisfying outcomes in all cases. Although viral and cellular factors have been extensively profiled to identify potential anti-COVID-19 targets, new drugs with significant efficacy remain to be developed. Here, we report the potent preclinical efficacy of ALD-R491, a vimentin-targeting small molecule compound, in treating COVID-19 through its host-directed antiviral and anti-inflammatory actions. We found that by altering the physical properties of vimentin filaments, ALD-491 affected general cellular processes as well as specific cellular functions relevant to SARS-CoV-2 infection. Specifically, ALD-R491 reduced endocytosis, endosomal trafficking, and exosomal release, thus impeding the entry and egress of the virus; increased the microcidal capacity of macrophages, thus facilitating the pathogen clearance; and enhanced the activity of regulatory T cells, therefore suppressing the overactive immune responses. In cultured cells, ALD-R491 potently inhibited the SARS-CoV-2 spike protein and human ACE2-mediated pseudoviral infection. In aged mice with ongoing, productive SARS-CoV-2 infection, ALD-R491 reduced disease symptoms as well as lung damage. In rats, ALD-R491 also reduced bleomycin-induced lung injury and fibrosis. Our results indicate a unique mechanism and significant therapeutic potential for ALD-R491 against COVID-19. We anticipate that ALD-R491, an oral, fast-acting, and non-cytotoxic agent targeting the cellular protein with multipart actions, will be convenient, safe, and broadly effective, regardless of viral mutations, for patients with early- or late-stage disease, post-COVID-19 complications, and other related diseases.
Related collections
Most cited references59

- Record: found
- Abstract: found
- Article: found
A pneumonia outbreak associated with a new coronavirus of probable bat origin
- Record: found
- Abstract: found
- Article: not found
Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China
- Record: found
- Abstract: found
- Article: not found