- Record: found
- Abstract: found
- Article: found
HDAC Inhibitors in Acute Myeloid Leukemia
Read this article at
Abstract
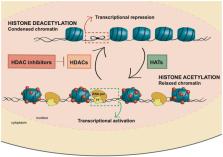
Acute myeloid leukemia (AML) is a hematological malignancy characterized by uncontrolled proliferation, differentiation arrest, and accumulation of immature myeloid progenitors. Although clinical advances in AML have been made, especially in young patients, long-term disease-free survival remains poor, making this disease an unmet therapeutic challenge. Epigenetic alterations and mutations in epigenetic regulators contribute to the pathogenesis of AML, supporting the rationale for the use of epigenetic drugs in patients with AML. While hypomethylating agents have already been approved in AML, the use of other epigenetic inhibitors, such as histone deacetylases (HDAC) inhibitors (HDACi), is under clinical development. HDACi such as Panobinostat, Vorinostat, and Tricostatin A have been shown to promote cell death, autophagy, apoptosis, or growth arrest in preclinical AML models, yet these inhibitors do not seem to be effective as monotherapies, but rather in combination with other drugs. In this review, we discuss the rationale for the use of different HDACi in patients with AML, the results of preclinical studies, and the results obtained in clinical trials. Although so far the results with HDACi in clinical trials in AML have been modest, there are some encouraging data from treatment with the HDACi Pracinostat in combination with DNA demethylating agents.
Related collections
Most cited references179
- Record: found
- Abstract: found
- Article: not found
How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers.
- Record: found
- Abstract: found
- Article: not found
Acute myeloid leukaemia.
- Record: found
- Abstract: found
- Article: not found