- Record: found
- Abstract: found
- Article: found
Multiple comorbid neuropathologies in the setting of Alzheimer's disease neuropathology and implications for drug development
Read this article at
Abstract
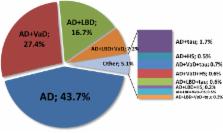
Dementia is often characterized as being caused by one of several major diseases, such as Alzheimer's disease (AD), cerebrovascular disease, Lewy body disease, or a frontotemporal degeneration. Failure to acknowledge that more than one entity may be present precludes attempts to understand interactive relationships. The clinicopathological studies of dementia demonstrate that multiple pathologic processes often coexist.
How overlapping pathologic findings affect the diagnosis and treatment of clinical AD and other dementia phenotypes was the topic taken up by the Alzheimer's Association's Research Roundtable in October 2014. This review will cover the neuropathologic basis of dementia, provide clinical perspectives on multiple pathologies, and discuss therapeutics and biomarkers targeting overlapping pathologies and how these issues impact clinical trials.High prevalence of multiple pathologic findings among individuals with clinical diagnosis of AD suggests that new treatment strategies may be needed to effectively treat AD and other dementing illnesses.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Staging TDP-43 pathology in Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found
Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease.
- Record: found
- Abstract: found
- Article: not found