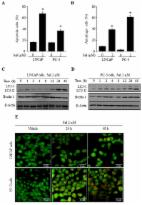
Recently, the interplay between autophagy and apoptosis has become an important factor in chemotherapy for cancer treatment. Inhibition of autophagy may be an effective strategy to improve the treatment of chemo-resistant cancer by consistent exposure to chemotherapeutic drugs. However, no reports have clearly elucidated the underlying mechanisms. Therefore, in this study, we assessed whether salinomycin, a promising anticancer drug, induces apoptosis and elucidated potential antitumor mechanisms in chemo-resistant prostate cancer cells. Cell viability assay, Western blot, annexin V/propidium iodide assay, acridine orange (AO) staining, caspase-3 activity assay, reactive oxygen species (ROS) production, and mitochondrial membrane potential were assayed. Our data showed that salinomycin alters the sensitivity of prostate cancer cells to autophagy. Pretreatment with 3-methyladenine (3-MA), an autophagy inhibitor, enhanced the salinomycin-induced apoptosis. Notably, salinomycin decreased phosphorylated of AKT and phosphorylated mammalian target of rapamycin (mTOR) in prostate cancer cells. Pretreatment with LY294002, an autophagy and PI3K inhibitor, enhanced the salinomycin-induced apoptosis by decreasing the AKT and mTOR activities and suppressing autophagy. However, pretreatment with PD98059 and SB203580, an extracellular signal-regulated kinases (ERK), and p38 inhibitors, suppressed the salinomycin-induced autophagy by reversing the upregulation of ERK and p38. In addition, pretreatment with N-acetyl-l-cysteine (NAC), an antioxidant, inhibited salinomycin-induced autophagy by suppressing ROS production. Our results suggested that salinomycin induces apoptosis, which was related to ROS-mediated autophagy through regulation of the PI3K/AKT/mTOR and ERK/p38 MAPK signaling pathways.