- Record: found
- Abstract: found
- Article: not found
Selective Coupling of Bioderived Aliphatic Alcohols with Acetone Using Hydrotalcite Derived Mg–Al Porous Metal Oxide and Raney Nickel
Read this article at
Abstract
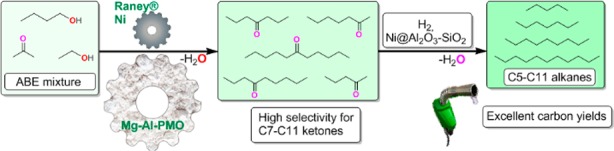
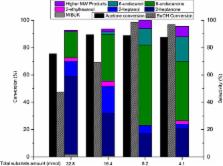
Fermentation of sugars to the so-called ABE mixture delivers a three component mixture of shorter chain oxygenates: acetone, n-butanol and ethanol. In order to convert these into liquid transportation fuels that are analogous to the currently used fossil energy carriers, novel catalytic chain elongation methods involving C–C bond formation are desired. Herein we report on a simple, non-noble-metal-based method for the highly selective coupling of 1-butanol and acetone into high molecular weight (C7–C11) ketones, as well as ABE mixtures into (C5–C11) ketones using the solid base Mg–Al–PMO in combination with small amount of Raney nickel. Upon hydrodeoxygenation, these ketones are converted to fuel range alkanes with excellent carbon utilization (up to 89%) using Earth abundant metal containing catalysis.
Abstract
Selective coupling of ABE mixture to chain elongated aliphatic ketones and their conversion into transportation fuel range alkanes is discussed.
Related collections
Most cited references39
- Record: found
- Abstract: not found
- Article: not found
Hydrolysis of lignocellulosic materials for ethanol production: a review
- Record: found
- Abstract: not found
- Article: not found
Catalytic conversion of biomass to biofuels
- Record: found
- Abstract: found
- Article: not found
