- Record: found
- Abstract: found
- Article: found
Stereodivergent synthesis via iridium-catalyzed asymmetric double allylic alkylation of cyanoacetate†

Read this article at
Abstract
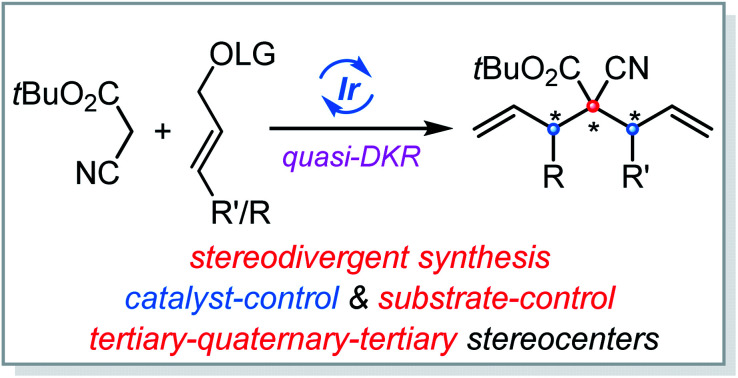
Methods that enable the rapid construction of multiple C–C bonds using a single catalyst with high diastereo- and enantio-control are particularly valuable in organic synthesis. Here, we report an Ir-catalyzed double allylic alkylation reaction in which bisnucleophilic cyanoacetate reacted successionally with electrophilic π-allyl-Ir species, producing various pseudo-C 2-symmetrical cyanoacetate derivatives in high yield with excellent stereocontrol. More challenging sequential allylic alkylation/allylic alkylation with two distinct allylic carbonates that can deliver the corresponding products bearing three contiguous tertiary–quaternary–tertiary stereocenters was also developed by using a modified catalytic system, which is revealed to be associated with the quasi-dynamic kinetic resolution of the initially formed diastereomeric monoallylation intermediates. Notably, stereodivergence for this sequential process depending on a single iridium catalyst was successfully realized, and up to six stereoisomers could be predictably prepared by combining the appropriate enantiomer of the chiral ligand for the iridium catalyst and adjusting the adding sequence of two distinct allylic precursors.
Abstract
Ir-catalyzed asymmetric double AAA reaction of cyanoacetate was developed, affording cyanoacetate derivatives in high yield with excellent stereocontrol. Notably, quasi-DKR is involved in the sequential protocol with two distinct allylic carbonates.
Related collections
Most cited references6
- Record: found
- Abstract: not found
- Book: not found
Classics in Stereoselective Synthesis
- Record: found
- Abstract: not found
- Book: not found
Comprehensive Asymmetric Catalysis
- Record: found
- Abstract: not found
- Book: not found