- Record: found
- Abstract: found
- Article: found
Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation
Read this article at
Abstract
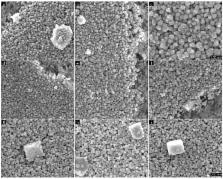
Electrically conductive films are key components in most printed and flexible electronics applications. For the solution processing of conductive films, inks containing silver nanoparticles (AgNPs) remain important because of their relatively easy processing and generally low resistivity after a sintering procedure. Because the commonly used, moderate sintering temperatures of 150–300 °C are still too high for most low-cost flexible substrates, expanding the knowledge of surface-ink interactions that affect the sintering temperature is desirable. It is known that chloride ions can assist the sintering of AgNP films by displacing capping agents on the surfaces of AgNPs. However, very little is known about other possible Cl-AgNP interactions that affect the resistivity and no interaction having the opposite effect (sintering inhibition) has been identified before. Here we identify such a Cl-AgNP interaction giving sintering inhibition and find that the mechanism involves the formation of AgCl nanocrystals within the AgNP film. The AgCl formation was observed after inkjet-printing of AgNP inks with polyvinylpyrrolidone (PVP) as the capping agent onto papers with quick-absorbing coatings containing 0.3 wt % KCl. Our findings show that chloride can have opposite roles during sintering, either assisting or inhibiting the sintering depending on the prevalence of AgCl formation. The prevalence of AgCl formation depends on the absorption properties and the capping agent.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions.
- Record: found
- Abstract: not found
- Article: not found
Intense pulsed light sintering of copper nanoink for printed electronics
- Record: found
- Abstract: not found
- Article: not found