- Record: found
- Abstract: found
- Article: found
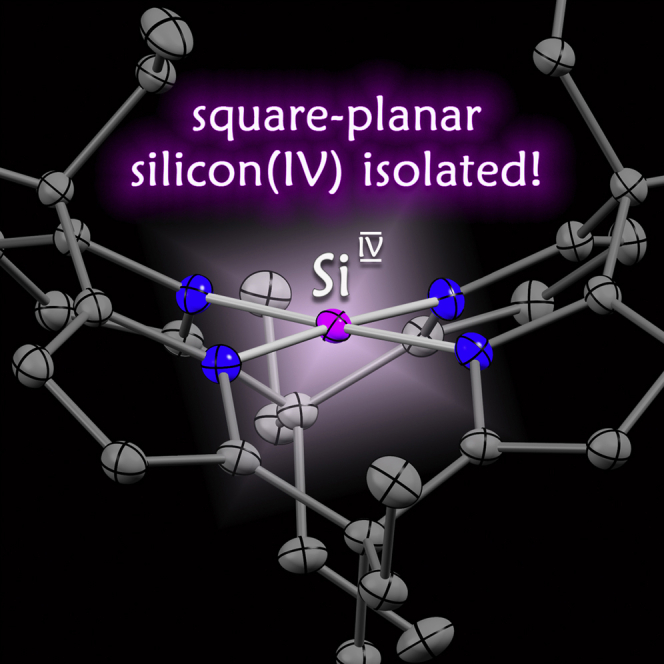
An isolable, crystalline complex of square-planar silicon(IV)
Read this article at
Summary
The structure and reactivity of silicon(IV), the second most abundant element in our Earth's crust, is determined by its invariant tetrahedral coordination geometry. Silicon(IV) with a square-planar configuration ( ptSi IV ) represents a transition state. Quantum theory supported the feasibility of stabilizing ptSi IV by structural constraint, but its isolation has not been achieved yet. Here, we present the synthesis and full characterization of the first square-planar coordinated silicon(IV). The planarity provokes an extremely low-lying unoccupied molecular orbital that induces unusual silicon redox chemistry and CH-agostic interactions. The small separation of the frontier molecular orbitals enables visible-light ligand-element charge transfer and bond-activation reactivity. Previously, such characteristics have been reserved for d-block metals or low-valent p-block elements. Planarization transfers them, for the first time, to a p-block element in the normal valence state.
Graphical abstract
Highlights
The bigger picture
Tetrahedral silicon(IV) compounds are the building blocks of our Earth’s crust. Here, we describe the first species of silicon(IV) with a square-planar configuration. The structural deformation has substantial consequences for the compounds’ physicochemical properties and imparts features usually associated with transition metals. Upon planarization, the frontier molecular orbital gap shrinks by more than 50% and enables ligand-element charge transfer, CH-bond agostic interactions, and spontaneous reactivity with inert bonds. Small frontier molecular orbital gaps are critical for bond-activation reactivity, catalysis, and photochemistry with transition metals. Traditional approaches to mimic these characteristics with the more abundant p-block elements rely on unusual valence or oxidation states. With the realization of square-planar silicon(IV), these peculiarities start reaching p-block elements in their natural oxidation states.
Abstract
Our Earth’s crust is covered with compounds of silicon(IV). In each, tetracoordinated silicon(IV) arranges its four substituents in a tetrahedral fashion, while other environments are unknown. This work describes the isolation and properties of a molecular complex with square-planar silicon(IV). The flattened structural motif provokes a range of features that primes the second most abundant element for new applications in catalysis and materials science.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found