- Record: found
- Abstract: found
- Article: found
Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate
Read this article at
Abstract
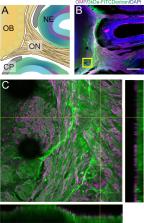
Cerebrospinal fluid (CSF) flows through the brain, transporting chemical signals and removing waste. CSF production in the brain is balanced by a constant outflow of CSF, the anatomical basis of which is poorly understood. Here, we characterized the anatomy and physiological function of the CSF outflow pathway along the olfactory sensory nerves through the cribriform plate, and into the nasal epithelia. Chemical ablation of olfactory sensory nerves greatly reduced outflow of CSF through the cribriform plate. The reduction in CSF outflow did not cause an increase in intracranial pressure (ICP), consistent with an alteration in the pattern of CSF drainage or production. Our results suggest that damage to olfactory sensory neurons (such as from air pollution) could contribute to altered CSF turnover and flow, providing a potential mechanism for neurological diseases.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
Intranasal delivery of biologics to the central nervous system.
- Record: found
- Abstract: found
- Article: not found
SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction.
- Record: found
- Abstract: found
- Article: not found