- Record: found
- Abstract: found
- Article: found
Gut microbiota contributes to bisphenol A-induced maternal intestinal and placental apoptosis, oxidative stress, and fetal growth restriction in pregnant ewe model by regulating gut-placental axis
Read this article at
Abstract
Background
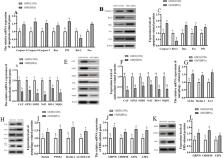
Bisphenol A (BPA) is an environmental contaminant with endocrine-disrupting properties that induce fetal growth restriction (FGR). Previous studies on pregnant ewes revealed that BPA exposure causes placental apoptosis and oxidative stress (OS) and decreases placental efficiency, consequently leading to FGR. Nonetheless, the response of gut microbiota to BPA exposure and its role in aggravating BPA-mediated apoptosis, autophagy, mitochondrial dysfunction, endoplasmic reticulum stress (ERS), and OS of the maternal placenta and intestine are unclear in an ovine model of gestation.
Results
Two pregnant ewe groups ( n = 8/group) were given either a subcutaneous (sc) injection of corn oil (CON group) or BPA (5 mg/kg/day) dissolved in corn oil (BPA group) once daily, from day 40 to day 110 of gestation. The maternal colonic digesta and the ileum and placental tissue samples were collected to measure the biomarkers of autophagy, apoptosis, mitochondrial dysfunction, ERS, and OS. To investigate the link between gut microbiota and the BPA-induced FGR in pregnant ewes, gut microbiota transplantation (GMT) was conducted in two pregnant mice groups ( n = 10/group) from day 0 to day 18 of gestation after removing their intestinal microbiota by antibiotics. The results indicated that BPA aggravates apoptosis, ERS and autophagy, mitochondrial function injury of the placenta and ileum, and gut microbiota dysbiosis in pregnant ewes. GMT indicated that BPA-induced ERS, autophagy, and apoptosis in the ileum and placenta are attributed to gut microbiota dysbiosis resulting from BPA exposure.
Conclusions
Our findings indicate the underlying role of gut microbiota dysbiosis and gut-placental axis behind the BPA-mediated maternal intestinal and placental apoptosis, OS, and FGR. The findings further provide novel insights into modulating the balance of gut microbiota through medication or probiotics, functioning via the gut-placental axis, to alleviate gut-derived placental impairment or FGR.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences
- Record: found
- Abstract: found
- Article: not found
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release.
- Record: found
- Abstract: found
- Article: not found