- Record: found
- Abstract: found
- Article: not found
The SUMO-specific isopeptidase SENP2 associates dynamically with nuclear pore complexes through interactions with karyopherins and the Nup107-160 nucleoporin subcomplex
Read this article at
Abstract
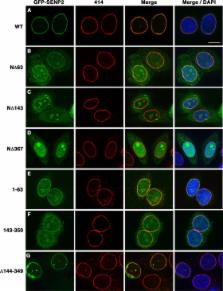
We determined that the small, ubiquitin-related modifier–specific isopeptidase, SENP2, is dynamically associated with nuclear pore complexes (NPCs). This association is determined by the activities of three N-terminal signals in SENP2: a high-affinity nuclear localization sequence, an Nup107-160–binding element, and a nuclear export signal. NPC association, and its potential regulation, affects SENP2 accessibility to substrates.
Abstract
The association of small, ubiquitin-related modifier–specific isopeptidases (also known as sentrin-specific proteases, or SENPs) with nuclear pore complexes (NPCs) is conserved in eukaryotic organisms ranging from yeast to mammals. However, the functional significance of this association remains poorly understood, particularly in mammalian cells. In this study, we have characterized the molecular basis for interactions between SENP2 and NPCs in human cells. Using fluorescence recovery after photobleaching, we demonstrate that SENP2, although concentrated at the nuclear basket, is dynamically associated with NPCs. This association is mediated by multiple targeting elements within the N-terminus of SENP2 that function cooperatively to mediate NPC localization. One of these elements consists of a high-affinity nuclear localization signal that mediates indirect tethering to FG-repeat–containing nucleoporins through karyopherins. A second element mediates interactions with the Nup107-160 nucleoporin subcomplex. A third element consists of a nuclear export signal. Collectively, our findings reveal that SENP2 is tethered to NPCs through a complex interplay of interactions with nuclear import and export receptors and nucleoporins. Disruption of these interactions enhances SENP2 substrate accessibility, suggesting an important regulatory node in the SUMO pathway.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
TANDEM: matching proteins with tandem mass spectra.
- Record: found
- Abstract: found
- Article: not found
