- Record: found
- Abstract: found
- Article: found
Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation
Read this article at
Abstract
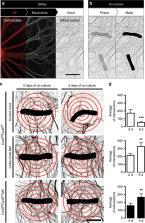
Denervation of skeletal muscles induces severe muscle atrophy, which is preceded by cellular alterations such as increased plasma membrane permeability, reduced resting membrane potential and accelerated protein catabolism. The factors that induce these changes remain unknown. Conversely, functional recovery following denervation depends on successful reinnervation. Here, we show that activation of nicotinic acetylcholine receptors (nAChRs) by quantal release of acetylcholine (ACh) from motoneurons is sufficient to prevent changes induced by denervation. Using in vitro assays, ACh and non-hydrolysable ACh analogs repressed the expression of connexin43 and connexin45 hemichannels, which promote muscle atrophy. In co-culture studies, connexin43/45 hemichannel knockout or knockdown increased innervation of muscle fibers by dorsal root ganglion neurons. Our results show that ACh released by motoneurons exerts a hitherto unknown function independent of myofiber contraction. nAChRs and connexin hemichannels are potential molecular targets for therapeutic intervention in a variety of pathological conditions with reduced synaptic neuromuscular transmission.
Abstract
Denervation of muscle fibres induces muscle atrophy, via mechanisms that remain unclear. Here, the authors show that binding of acetylcoline to its receptor at the neuromuscular junction represses the expression of connexins 43 and 45, which promote atrophy, and is sufficient to prevent denervation-induced loss of myofibre mass.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels.
- Record: found
- Abstract: found
- Article: not found
MIR-206 regulates connexin43 expression during skeletal muscle development
- Record: found
- Abstract: found
- Article: found