- Record: found
- Abstract: found
- Article: found
Mechanisms of Action of Adjuvants
Read this article at
Abstract
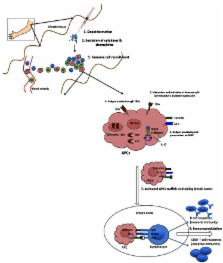
Adjuvants are used in many vaccines, but their mechanisms of action are not fully understood. Studies from the past decade on adjuvant mechanisms are slowly revealing the secrets of adjuvant activity. In this review, we have summarized the recent progress in our understanding of the mechanisms of action of adjuvants. Adjuvants may act by a combination of various mechanisms including formation of depot, induction of cytokines and chemokines, recruitment of immune cells, enhancement of antigen uptake and presentation, and promoting antigen transport to draining lymph nodes. It appears that adjuvants activate innate immune responses to create a local immuno-competent environment at the injection site. Depending on the type of innate responses activated, adjuvants can alter the quality and quantity of adaptive immune responses. Understanding the mechanisms of action of adjuvants will provide critical information on how innate immunity influences the development of adaptive immunity, help in rational design of vaccines against various diseases, and can inform on adjuvant safety.
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
The inflammasomes: guardians of the body.
- Record: found
- Abstract: found
- Article: not found
Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection.
- Record: found
- Abstract: found
- Article: not found
The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
