- Record: found
- Abstract: found
- Article: found
Serum IL-12 Is Increased in Mexican Obese Subjects and Associated with Low-Grade Inflammation and Obesity-Related Parameters
Read this article at
Abstract
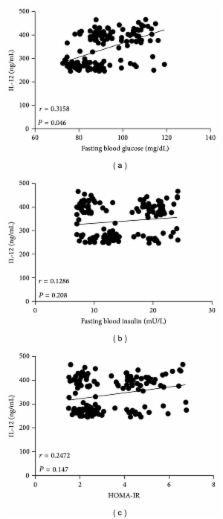
Interleukin-(IL-) 12 has been recently suggested to participate during development of insulin resistance in obese mice. Nevertheless, serum IL-12 levels have not been accurately determined in overweight and obese humans. We thus studied serum concentrations of IL-12 in Mexican adult individuals, examining their relationship with low-grade inflammation and obesity-related parameters. A total of 147 healthy individuals, 43 normal weight, 61 overweight, and 43 obese subjects participated in the study. Circulating levels of IL-12, tumor necrosis factor-alpha (TNF- α ), leptin, insulin, glucose, total cholesterol, and triglyceride were measured after overnight fasting in all of the study subjects. Waist circumference and body fat percentage were recorded for all the participants. Serum IL-12 was significantly higher in overweight and obese individuals than in normal weight controls. Besides being strongly related with body mass index ( r = 0.5154), serum IL-12 exhibited a significant relationship with abdominal obesity ( r = 0.4481), body fat percentage ( r = 0.5625), serum glucose ( r = 0.3158), triglyceride ( r = 0.3714), and TNF- α ( r = 0.4717). Thus, serum levels of IL-12 are increased in overweight and obese individuals and show a strong relationship with markers of low-grade inflammation and obesity in the Mexican adult population. Further research is needed to understand the role of IL-12 in developing obesity-associated alterations in humans.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The link between abdominal obesity, metabolic syndrome and cardiovascular disease.
- Record: found
- Abstract: found
- Article: not found
Insulin resistance associated to obesity: the link TNF-alpha.
- Record: found
- Abstract: found
- Article: not found