- Record: found
- Abstract: found
- Article: found
S-acylation of SOD1, CCS, and a stable SOD1-CCS heterodimer in human spinal cords from ALS and non-ALS subjects
Read this article at
Abstract
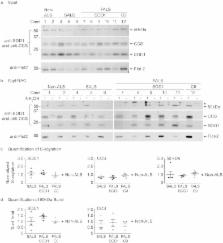
Previously, we found that human Cu, Zn-superoxide dismutase (SOD1) is S-acylated (palmitoylated) in vitro and in amyotrophic lateral sclerosis (ALS) mouse models, and that S-acylation increased for ALS-causing SOD1 mutants relative to wild type. Here, we use the acyl resin-assisted capture (acyl-RAC) assay to demonstrate S-acylation of SOD1 in human post-mortem spinal cord homogenates from ALS and non-ALS subjects. Acyl-RAC further revealed that endogenous copper chaperone for SOD1 (CCS) is S-acylated in both human and mouse spinal cords, and in vitro in HEK293 cells. SOD1 and CCS formed a highly stable heterodimer in human spinal cord homogenates that was resistant to dissociation by boiling, denaturants, or reducing agents and was not observed in vitro unless both SOD1 and CCS were overexpressed. Cysteine mutations that attenuate SOD1 maturation prevented the SOD1-CCS heterodimer formation. The degree of S-acylation was highest for SOD1-CCS heterodimers, intermediate for CCS monomers, and lowest for SOD1 monomers. Given that S-acylation facilitates anchoring of soluble proteins to cell membranes, our findings suggest that S-acylation and membrane localization may play an important role in CCS-mediated SOD1 maturation. Furthermore, the highly stable S-acylated SOD1-CCS heterodimer may serve as a long-lived maturation intermediate in human spinal cord.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Superoxide radical and superoxide dismutases.
- Record: found
- Abstract: found
- Article: not found
Site-specific analysis of protein S-acylation by resin-assisted capture.
- Record: found
- Abstract: found
- Article: not found