- Record: found
- Abstract: found
- Article: found
A Combined Digital and Biomarker Diagnostic Aid for Mood Disorders (the Delta Trial): Protocol for an Observational Study
Abstract
Background
Mood disorders affect hundreds of millions of people worldwide, imposing a substantial medical and economic burden. Existing diagnostic methods for mood disorders often result in a delay until accurate diagnosis, exacerbating the challenges of these disorders. Advances in digital tools for psychiatry and understanding the biological basis of mood disorders offer the potential for novel diagnostic methods that facilitate early and accurate diagnosis of patients.
Objective
The Delta Trial was launched to develop an algorithm-based diagnostic aid combining symptom data and proteomic biomarkers to reduce the misdiagnosis of bipolar disorder (BD) as a major depressive disorder (MDD) and achieve more accurate and earlier MDD diagnosis.
Methods
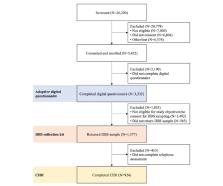
Participants for this ethically approved trial were recruited through the internet, mainly through Facebook advertising. Participants were then screened for eligibility, consented to participate, and completed an adaptive digital questionnaire that was designed and created for the trial on a purpose-built digital platform. A subset of these participants was selected to provide dried blood spot (DBS) samples and undertake a World Health Organization World Mental Health Composite International Diagnostic Interview (CIDI). Inclusion and exclusion criteria were chosen to maximize the safety of a trial population that was both relevant to the trial objectives and generalizable. To provide statistical power and validation sets for the primary and secondary objectives, 840 participants were required to complete the digital questionnaire, submit DBS samples, and undertake a CIDI.
Results
The Delta Trial is now complete. More than 3200 participants completed the digital questionnaire, 924 of whom also submitted DBS samples and a CIDI, whereas a total of 1780 participants completed a 6-month follow-up questionnaire and 1542 completed a 12-month follow-up questionnaire. The analysis of the trial data is now underway.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI)

- Record: found
- Abstract: found
- Article: found
What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies
- Record: found
- Abstract: found
- Article: not found