- Record: found
- Abstract: found
- Article: found
Flame Synthesis of Cu/ZnO–CeO 2 Catalysts: Synergistic Metal–Support Interactions Promote CH 3OH Selectivity in CO 2 Hydrogenation
Read this article at
Abstract

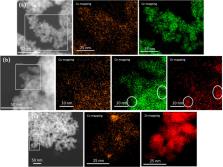
The hydrogenation of CO 2 to CH 3OH is an important reaction for future renewable energy scenarios. Herein, we compare Cu/ZnO, Cu/CeO 2, and Cu/ZnO–CeO 2 catalysts prepared by flame spray pyrolysis. The Cu loading and support composition were varied to understand the role of Cu–ZnO and Cu–CeO 2 interactions. CeO 2 addition improves Cu dispersion with respect to ZnO, owing to stronger Cu–CeO 2 interactions. The ternary Cu/ZnO–CeO 2 catalysts displayed a substantially higher CH 3OH selectivity than binary Cu/CeO 2 and Cu/ZnO catalysts. The high CH 3OH selectivity in comparison with a commercial Cu–ZnO catalyst is also confirmed for Cu/ZnO–CeO 2 catalyst prepared with high Cu loading (∼40 wt %). In situ IR spectroscopy was used to probe metal–support interactions in the reduced catalysts and to gain insight into CO 2 hydrogenation over the Cu–Zn–Ce oxide catalysts. The higher CH 3OH selectivity can be explained by synergistic Cu–CeO 2 and Cu–ZnO interactions. Cu–ZnO interactions promote CO 2 hydrogenation to CH 3OH by Zn-decorated Cu active sites. Cu–CeO 2 interactions inhibit the reverse water–gas shift reaction due to a high formate coverage of Cu and a high rate of hydrogenation of the CO intermediate to CH 3OH. These insights emphasize the potential of fine-tuning metal–support interactions to develop improved Cu-based catalysts for CO 2 hydrogenation to CH 3OH.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts.
- Record: found
- Abstract: not found
- Article: not found
Beyond oil and gas: the methanol economy.
- Record: found
- Abstract: found
- Article: not found