- Record: found
- Abstract: found
- Article: not found
Metabolic reprogramming by the S-nitroso-CoA Reductase system protects from kidney injury
Read this article at
Abstract
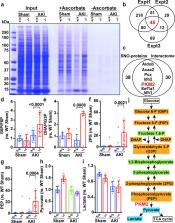
Endothelial nitric oxide (NO) synthase (eNOS) is protective against kidney injury, but the molecular mechanisms are poorly understood 1, 2 . NO-based cellular signaling is generally mediated by protein S-nitrosylation, the oxidative modification of Cys residues to form S-nitrosothiols (SNOs). S-nitrosylation regulates proteins in all functional classes, and is controlled by enzymatic machinery including S-nitrosylases and denitrosylases that add and remove SNO from proteins, respectively 3, 4 . We recently reported in Saccharomyces cerevisiae that the classic metabolic intermediate Co-enzymeA (CoA) serves as an endogenous source of SNOs through its conjugation with NO to form S-nitroso-CoA (SNO-CoA), and that S-nitrosylation of proteins by SNO-CoA is governed by its cognate denitrosylase, SNO-CoA reductase (SCoR) 5 . Mammals possess a functional homologue of yeast SCoR, an aldo-keto reductase family member (AKR1A1) 5 with an unknown physiological role. Here we report that the SNO-CoA/AKR1A1 (SCoR) system is highly expressed in renal proximal tubules where it transduces the activity of eNOS in reprogramming intermediary metabolism, thereby protecting kidneys from acute kidney injury (AKI). Specifically, AKR1A1 deletion in mice to reduce SCoR activity increased protein S-nitrosylation, protected against AKI and improved survival, whereas renoprotection was lost in Akr1a1 −/− / eNOS −/− mice. Metabolic profiling coupled with unbiased mass spectrometry-based SNO-protein identification revealed that protection by the SNO-CoA/SCoR system is mediated by inhibitory S-nitrosylation of pyruvate kinase M2 (PKM2) through a novel locus of regulation, thereby balancing fuel utilization (through glycolysis) with redox protection (through the pentose phosphate shunt). Targeted deletion of PKM2 from mouse proximal tubules recapitulated precisely the protective and mechanistic effects of S-nitrosylation in Akr1a1 −/− mice, whereas Cys-mutant PKM2 refractory to S-nitrosylation negated SNO-CoA bioactivity. Our discoveries provide a first physiological function of the SNO-CoA/SCoR system in mammals, reveal novel regulation of renal metabolism and of PKM2 in differentiated tissues in particular, and offer a new perspective on kidney injury with therapeutic implications.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Pathophysiology of ischemic acute kidney injury.
- Record: found
- Abstract: found
- Article: not found
Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis
- Record: found
- Abstract: found
- Article: not found
