- Record: found
- Abstract: found
- Article: found
Radiation‐induced effects on TGF‐β and PDGF receptor signaling in cancer‐associated fibroblasts
Read this article at
Abstract
Background
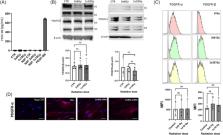
Cancer‐associated fibroblasts (CAFs) consist of heterogeneous connective tissue cells and are often constituting the most abundant cell type in the tumor stroma. Radiation effects on tumor stromal components like CAFs in the context of radiation treatment is not well‐described.
Aim
This study explores potential changes induced by ionizing radiation (IR) on platelet‐derived growth factor (PDGF)/PDGFRs and transforming growth factor‐beta (TGF‐β)/TGFβRs signaling systems in CAFs.
Methods and Results
Experiments were carried out by employing primary cultures of human CAFs isolated from freshly resected non‐small cell lung carcinoma tumor tissues. CAF cultures from nine donors were treated with one high (1 × 18 Gy) or three fractionated (3 × 6 Gy) radiation doses. Alterations in expression levels of TGFβRII and PDGFRα/β induced by IR were analyzed by western blots and flow cytometry. In the presence or absence of cognate ligands, receptor activation was studied in nonirradiated and irradiated CAFs. Radiation exposure did not exert changes in expression of PDGF or TGF‐β receptors in CAFs. Additionally, IR alone was unable to trigger activation of either receptor. The radiation regimens tested did not affect PDGFRβ signaling in the presence of PDGF‐BB. In contrast, signaling via pSmad2/3 and pSmad1/5/8 appeared to be down‐regulated in irradiated CAFs after stimulation with TGF‐β, as compared with controls.
Conclusion
Our data demonstrate that IR by itself is insufficient to induce measurable changes in PDGF or TGF‐β receptor expression levels or to induce receptor activation in CAFs. However, in the presence of their respective ligands, exposure to radiation at certain doses appear to interfere with TGF‐β receptor signaling.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells
- Record: found
- Abstract: found
- Article: not found