- Record: found
- Abstract: found
- Article: found
Isolation and characterization of bovine herpesvirus 4 (BoHV-4) from a cow affected by post partum metritis and cloning of the genome as a bacterial artificial chromosome
Read this article at
Abstract
Background
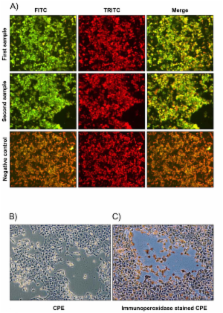
Bovine herpesvirus 4 (BoHV-4) is a gammaherpesvirus with a Worldwide distribution in cattle and is often isolated from the uterus of animals with postpartum metritis or pelvic inflammatory disease. Virus strain adaptation to an organ, tissue or cell type is an important issue for the pathogenesis of disease. To explore the mechanistic role of viral strain variation for uterine disease, the present study aimed to develop a tool enabling precise genetic discrimination between strains of BoHV-4 and to easily manipulate the viral genome.
Methods
A strain of BoHV-4 was isolated from the uterus of a persistently infected cow and designated BoHV-4-U. The authenticity of the isolate was confirmed by RFLP-PCR and sequencing using the TK and IE2 loci as genetic marker regions for the BoHV-4 genome. The isolated genome was cloned as a Bacterial Artificial Chromosome (BAC) and manipulated through recombineering technology
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle.
- Record: found
- Abstract: found
- Article: not found
Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells.
- Record: found
- Abstract: found
- Article: not found