- Record: found
- Abstract: found
- Article: found
Late complications of transcatheter atrial septal defect closure requiring urgent surgery
brief-report
Pavle Kovacevic
1
,
2 ,
Ilija Srdanovic
1
,
2 ,
Vladimir Ivanovic
1
,
2 ,
Jovan Rajic
2 ,
Nemanja Petrovic
2 ,
Lazar Velicki
1
,
2
,
29 November 2017
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Transcatheter atrial septal defect (ASD) closure has become a widely applied procedure
– the recommended method of therapy in eligible patients due to the improved learning
curve, cosmetic benefits and shorter recovery time [1]. Although the performance and
safety of these devices appear to be reliable, certain risks and complications remain.
Case reports
Case 1
A 21-year-old female patient was transferred to our hospital after being diagnosed
with massive pulmonary thromboembolism (PTE). Three years prior to admission, she
underwent transcatheter closure of the secundum ASD with a 33 mm CardioSEAL-StarFLEX
occluder (NMT Medical, Boston, MA, USA). A year ago, she was involved in a car accident
and sustained significant blunt chest trauma. Transthoracic echocardiography confirmed
the presence of thrombi in the right atrium and the pulmonary artery, with massive
dilatation of the right ventricle and the pulmonary artery, along with severe pulmonary
hypertension. Also, protrusion or dislodgement of the occluder was suspected. Her
deteriorated clinical conditions warranted immediate surgery. The patient was put
on a cardiopulmonary bypass (CPB) and the right atrium and the pulmonary artery were
opened. Several thrombi were removed, the largest being 2 × 3 cm. The ASD occluder
was identified with a thrombus attached to it and evident device-arm fracture (Figure
1). The occluder underwent almost complete healing with full endocardium covering
except in the rim area. The device was removed and the ASD was repaired with a patch.
Unfortunately, due to right heart failure, the patient could not be successfully weaned
from the CPB, not even after an artificially created interatrial shunt, and she expired.
Figure 1
A, B – Trans-esophageal echocardiography demonstrating a mass in the right atrium
and detached device, C, D – intraoperative finding
Although one cannot say with absolute certainty that massive PTE developed because
of device-related thrombosis, it seems intuitive that blood turbulence around the
protruded umbrella and device-arm fracture could have acted as a nidus for repeated
thrombus formation with subsequent embolization. The occluder malfunction (fracture)
was most likely the result of sustained blunt chest trauma a year prior to admission.
We hypothesize that the sudden increase in intrathoracic pressure during trauma as
well as direct compression on the heart generated a point of high wall stress around
the occluder’s septal insertion, which may have led to device fracture and dislodgment.
Case 2
A 32-year-old female patient was referred to our hospital with the diagnosis of acute
aortic dissection. She complained of a sharp tearing pain in the chest and back with
a loss of consciousness. A year prior to admission, she underwent ASD closure with
a 26 mm Amplatzer occluder (St. Jude Medical, Minneapolis, MN, USA) in another hospital.
Chest computed tomography was performed showing massive pericardial effusion and no
clear origin of contrast extravasation, and no signs of intimal flap throughout the
aorta. Due to a cardiac tamponade and deteriorated clinical conditions, the patient
was rushed to surgery where she arrested during anesthesia induction and recovered
upon pericardial incision and evacuation of 400 ml of blood. She was put on a CPB
and the aortic root erosion in the region of non-coronary cusp was identified (Figure
2). No signs of other aortic pathology (dissection) were evident. The tear was sutured
with two pledgeted sutures. The right atrium was opened with no signs of thrombi.
The Amplatzer occluder was in close relation to the roof of the atria, and consequently
the aortic root, and was undoubtedly the cause of the aortic perforation. It was extracted
and the ASD was repaired with a patch. She made an uneventful recovery and was discharged
home on the 8th postoperative day.
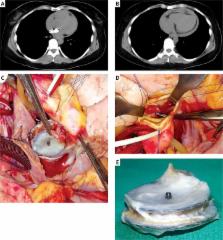
Figure 2
A, B – Computerized tomography showing the position of the device and pericardial
effusion, C – extraction of Amplatzer device, D – aortic root erosion, E – extracted
device
Discussion
Transcatheter closure of ASD is the recommended method of treatment in patients with
suitable defect anatomy (class I) [2, 3]. Due to inherent design properties (size,
shape, material, and construction), every device is associated with a specific type
of complications, which are potentially life threatening. Complications commonly associated
with ASD closure device include residual shunts, embolization, device-related thrombosis,
erosion and perforation of the heart, infective endocarditis, and sudden death.
Perforation is the most feared complication described in the literature with the incidence
of device erosion in the United States around 0.1% [4]. Perforation is most likely
to happen during the first 48 h after the procedure and it is rarely manifested as
a late complication. Perforation usually develops on the atrial dome and the adjacent
aorta. Patients can present with haemopericardium, pericardial effusion, cardiovascular
collapse, and sudden cardiac death. The U.S. Food and Drug Administration issued a
warning about safety issues encountered with Amplatzer septal occluder devices stating
that these devices may cause life-threatening tissue erosion inside the heart requiring
immediate surgery. Several mechanisms on how the device may lead to perforation were
proposed [4]. Deficient rims in vulnerable areas could increase the chance of contact
between the device and the atrial wall in the same manner as the oversizing of a device.
Device-related thrombosis has been reported in many series with the incidence of thrombus
formation of 1.2% in ASD patients [5]. The incidence of thrombus formation is highest
during the first 4 weeks after device implantation and is extremely rare after 8–12
months. The specific design of the CardioSEAL-StarFLEX device (a metallic framework
with Dacron fabric) rendered them prone to thrombosis as well as stress-mediated device
arm fractures. The case of late thrombus formation demonstrated in our patient occurred
following major blunt chest trauma that might have led to device-arm fracture that,
in turn, could have acted as a nidus for repeated thrombus formation. Specifically,
the majority of thrombi were found around the rim area of the occluder (fractured
wires) – an area with no complete healing and endothelialization. Usually, thrombi
resolve spontaneously after anticoagulation therapy with heparin or warfarin, although
some thrombi require surgical intervention, as demonstrated in our case. A longer
period of surveillance after device ASD closure might be warranted in order to capture
late occurrence of device malfunction, which may be associated with thromboembolic
events.
Conflict of interest
The authors declare no conflict of interest.
Related collections
Most cited references5
- Record: found
- Abstract: found
- Article: not found
Early and late complications associated with transcatheter occlusion of secundum atrial septal defect.
Massimo Chessa, Mario Carminati, Gianfranco Butera … (2002)
- Record: found
- Abstract: found
- Article: not found
Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients.
Horst Sievert, Thomas Trepels, Elisabeth Zadan … (2004)
- Record: found
- Abstract: found
- Article: not found
Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk.
S Kleinman, J. Bass, Ziyad M Hijazi … (2004)