- Record: found
- Abstract: found
- Article: found
Therapeutic Potential of Sclareol in Experimental Models of Rheumatoid Arthritis
Read this article at
Abstract
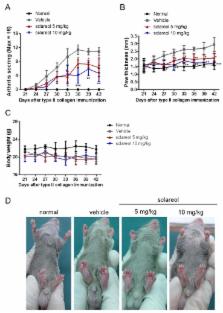
Previous studies have shown that the natural diterpene compound, sclareol, potentially inhibits inflammation, but it has not yet been determined whether sclareol can alleviate inflammation associated with rheumatoid arthritis (RA). Here, we utilized human synovial cell line, SW982, and an experimental murine model of rheumatoid arthritis, collagen-induced arthritis (CIA), to evaluate the therapeutic effects of sclareol in RA. Arthritic DBA/1J mice were dosed with 5 and 10 mg/kg sclareol intraperitoneally every other day over 21 days. Arthritic severity was evaluated by levels of anti-collagen II (anti-CII) antibody, inflammatory cytokines, and histopathologic examination of knee joint tissues. Our results reveal that the serum anti-CII antibody, cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and IL-17, as well as Th17 and Th1 cell population in inguinal lymph nodes, were significantly lower in sclareol-treated mice compared to the control group. Also, the sclareol treatment groups showed reduced swelling in the paws and lower histological arthritic scores, indicating that sclareol potentially mitigates collagen-induced arthritis. Furthermore, IL-1β-stimulated SW982 cells secreted less inflammatory cytokines (TNF-α and IL-6), which is associated with the downregulation of p38-mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and NF-κB pathways. Overall, we demonstrate that sclareol could relieve arthritic severities by modulating excessive inflammation and our study merits the pharmaceutical development of sclareol as a therapeutic treatment for inflammation associated with RA.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis.
- Record: found
- Abstract: found
- Article: found
Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis
- Record: found
- Abstract: found
- Article: found