- Record: found
- Abstract: found
- Article: found
Enhancement of Force Generated by Individual Myosin Heads in Skinned Rabbit Psoas Muscle Fibers at Low Ionic Strength
Read this article at
Abstract
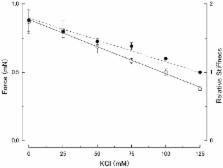
Although evidence has been presented that, at low ionic strength, myosin heads in relaxed skeletal muscle fibers form linkages with actin filaments, the effect of low ionic strength on contraction characteristics of Ca 2+-activated muscle fibers has not yet been studied in detail. To give information about the mechanism of muscle contraction, we have examined the effect of low ionic strength on the mechanical properties and the contraction characteristics of skinned rabbit psoas muscle fibers in both relaxed and maximally Ca 2+-activated states. By progressively decreasing KCl concentration from 125 mM to 0 mM (corresponding to a decrease in ionic strength μ from 170 mM to 50 mM), relaxed fibers showed changes in mechanical response to sinusoidal length changes and ramp stretches, which are consistent with the idea of actin-myosin linkage formation at low ionic strength. In maximally Ca 2+-activated fibers, on the other hand, the maximum isometric force increased about twofold by reducing KCl concentration from 125 to 0 mM. Unexpectedly, determination of the force-velocity curves indicated that, the maximum unloaded shortening velocity V max, remained unchanged at low ionic strength. This finding indicates that the actin-myosin linkages, which has been detected in relaxed fibers at low ionic strength, are broken quickly on Ca 2+ activation, so that the linkages in relaxed fibers no longer provide any internal resistance against fiber shortening. The force-velocity curves, obtained at various levels of steady Ca 2+-activated isometric force, were found to be identical if they are normalized with respect to the maximum isometric force. The MgATPase activity of muscle fibers during isometric force generation was found not to change appreciably at low ionic strength despite the two-fold increase in Ca 2+-activated isometric force. These results can be explained in terms of enhancement of force generated by individual myosin heads, but not by any changes in kinetic properties of cyclic actin-myosin interaction.
Related collections
Most cited references19
- Record: found
- Abstract: not found
- Article: not found
Mechanism of adenosine triphosphate hydrolysis by actomyosin.
- Record: found
- Abstract: found
- Article: not found
X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle.
- Record: found
- Abstract: found
- Article: not found