- Record: found
- Abstract: found
- Article: found
A virtuous cycle operated by ERp44 and ERGIC-53 guarantees proteostasis in the early secretory compartment

Read this article at
Summary
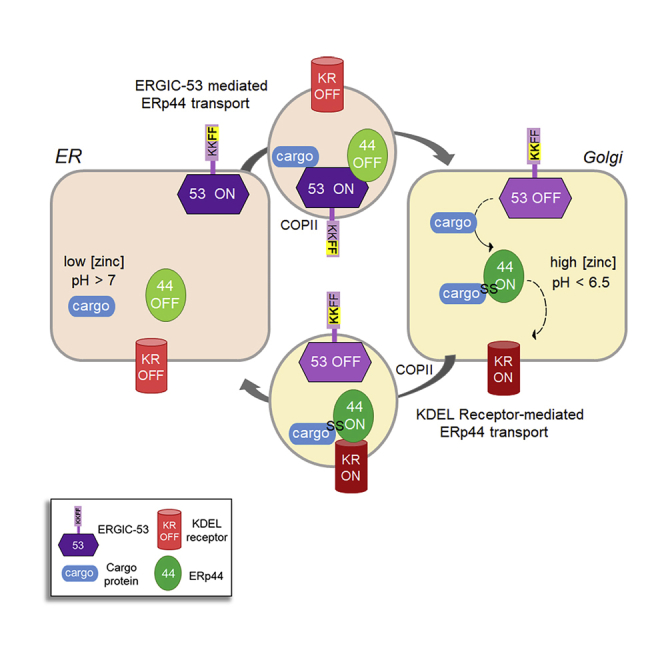
The composition of the secretome depends on the combined action of cargo receptors that facilitate protein transport and sequential checkpoints that restrict it to native conformers. Acting after endoplasmic reticulum (ER)-resident chaperones, ERp44 retrieves its clients from downstream compartments. To guarantee efficient quality control, ERp44 should exit the ER as rapidly as its clients, or more. Here, we show that appending ERp44 to different cargo proteins increases their secretion rates. ERp44 binds the cargo receptor ER-Golgi intermediate compartment (ERGIC)-53 in the ER to negotiate preferential loading into COPII vesicles. Silencing ERGIC-53, or competing for its COPII binding with 4-phenylbutyrate, causes secretion of Prdx4, an enzyme that relies on ERp44 for intracellular localization. In more acidic, zinc-rich downstream compartments, ERGIC-53 releases its clients and ERp44, which can bind and retrieve non-native conformers via KDEL receptors. By coupling the transport of cargoes and inspector proteins, cells ensure efficiency and fidelity of secretion.
Graphical abstract
Highlights
Abstract
Biological Sciences; Biochemistry; Molecular Biology; Cell Biology
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins.
- Record: found
- Abstract: found
- Article: not found
Protein quality control in the early secretory pathway.

- Record: found
- Abstract: found
- Article: found