- Record: found
- Abstract: found
- Article: found
Self-Reported Effectiveness and Safety of Trokie ® Lozenges: A Standardized Formulation for the Buccal Delivery of Cannabis Extracts
Read this article at
Abstract
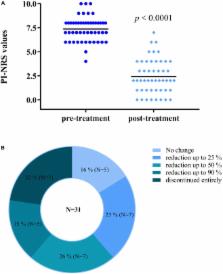
Therapeutic use of cannabinoids, the main active ingredients of Cannabis sativa L., is often hindered by their limited bioavailability and undesirable psychoactivity. We conducted an observational study in December 2016 and another one in February 2018 to investigate respectively: (i) the effectiveness of Trokie ® lozenges, a standardized formulation containing cannabis extracts, to deliver cannabinoids via buccal absorption and (ii) its long-term safety. Participants were members of the Palliative Care Corporation health clinic, registered California cannabis patients, and had a diagnosis of chronic non-cancer pain. For the effectiveness study, 49 participants were asked to self-report pain perception before and after 1–12 weeks of taking Trokie ® lozenges, using an 11-point pain intensity numeric rating scale (PI-NRS). A mean reduction in PI-NRS score of 4.9 ± 2.0 points was observed. Onset of analgesia typically varied between 5 and 40 min, which seems consistent with, at least partial, buccal absorption. In the safety study, 35 participants were asked to complete a questionnaire about adverse events (AEs) associated with Trokie ® lozenges. AEs were reported by 16 subjects (46%), the most common being dizziness/unsteadiness ( N = 7), bad taste ( N = 5), and throat irritation/dry mouth ( N = 4). None of the self-reported AEs resulted in a serious medical situation and most of them had limited impact on daily functions. Despite the AEs, 90% of participants reported being “satisfied” or “very satisfied” with the product. These observations suggest that buccal administration of standardized extracts via Trokie ® lozenges may represent an efficacious and safe approach to cannabis administration.
Related collections
Most cited references14
- Record: found
- Abstract: not found
- Article: not found
Practical considerations in medical cannabis administration and dosing
- Record: found
- Abstract: found
- Article: not found
PEGylation for enhancing nanoparticle diffusion in mucus.
- Record: found
- Abstract: found
- Article: not found