- Record: found
- Abstract: found
- Article: found
Antibiotic treatment modulates protein components of cytotoxic outer membrane vesicles of multidrug-resistant clinical strain, Acinetobacter baumannii DU202
Read this article at
Abstract
Background
Outer membrane vesicles (OMVs) of Acinetobacter baumannii are cytotoxic and elicit a potent innate immune response. OMVs were first identified in A. baumannii DU202, an extensively drug-resistant clinical strain. Herein, we investigated protein components of A. baumannii DU202 OMVs following antibiotic treatment by proteogenomic analysis.
Methods
Purified OMVs from A. baumannii DU202 grown in different antibiotic culture conditions were screened for pathogenic and immunogenic effects, and subjected to quantitative proteomic analysis by one-dimensional electrophoresis and liquid chromatography combined with tandem mass spectrometry (1DE-LC-MS/MS). Protein components modulated by imipenem were identified and discussed.
Results
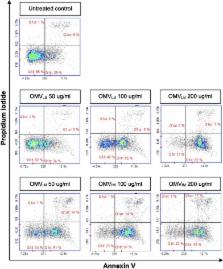
OMV secretion was increased > twofold following imipenem treatment, and cytotoxicity toward A549 human lung carcinoma cells was elevated. A total of 277 proteins were identified as components of OMVs by imipenem treatment, among which β-lactamase OXA-23, various proteases, outer membrane proteins, β-barrel assembly machine proteins, peptidyl-prolyl cis–trans isomerases and inherent prophage head subunit proteins were significantly upregulated.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells.
- Record: found
- Abstract: found
- Article: not found
Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion.
- Record: found
- Abstract: found
- Article: found
Acinetobacter baumannii Secretes Cytotoxic Outer Membrane Protein A via Outer Membrane Vesicles
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.