- Record: found
- Abstract: found
- Article: found
Formation of circular polyribosomes on eukaryotic mRNA without cap-structure and poly(A)-tail: a cryo electron tomography study
Read this article at
Abstract
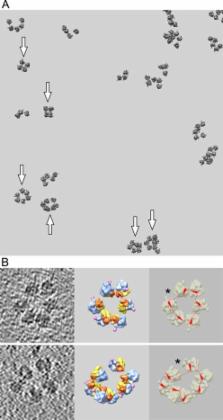
The polyribosomes newly formed on recombinant GFP-encoding mRNAs in a wheat germ cell-free translation system were analyzed using cryo-electron tomography, with sub-tomogram averaging of polysomal ribosomes and reconstruction of 3D structures of individual polyribosomes. The achieved level of resolution in the reconstructed polyribosomes allowed deducing the mRNA path by connecting adjacent exit and entry sites at the ribosomes inside each polyribosome. In this way, the circularity of a significant fraction (about 50%) of translating polyribosomes was proved in the case of the capped poly(A)-tailed mRNA, in agreement with the existing paradigm of the circularization via interaction of cap-bound initiation factor eIF4F with poly(A)-binding protein. However, translation of the capped mRNA construct without poly(A) tail, but with unspecific 3′-UTR derived from non-coding plasmid sequence, also led to the formation of circular polyribosomes in similar proportion (40%). Moreover, the polyribosomes formed on the uncapped non-polyadenylated mRNA with non-synergistic 5′- and 3′-UTRs proved to be circular as well, and appeared in the same proportion as in the previous cases. Thus, the formation of circular polyribosomes was found to be virtually independent of the presence of cap structure and poly(A) tail in mRNA, in contrast to the longstanding paradigm in the field.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Circularization of mRNA by eukaryotic translation initiation factors.
- Record: found
- Abstract: found
- Article: not found
XMIPP: a new generation of an open-source image processing package for electron microscopy.
- Record: found
- Abstract: found
- Article: not found