- Record: found
- Abstract: found
- Article: found
Tumor Microenvironment Modulation by Neoadjuvant Erlotinib Therapy and Its Clinical Impact on Operable EGFR-Mutant Non–Small Cell Lung Cancer
Read this article at
Abstract
Purpose
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors have greatly improved survival in EGFR-mutant (EGFRm) non–small cell lung cancer (NSCLC); however, their effects on the tumor microenvironment (TME) are unknown. We assessed the changes induced by neoadjuvant erlotinib therapy (NE) in the TME of operable EGFRm NSCLC.
Materials and Methods
This was a single-arm phase II trial for neoadjuvant/adjuvant erlotinib therapy in patients with stage II/IIIA EGFRm NSCLC ( EGFR exon 19 deletion or L858R mutations). Patients received up to 2 cycles of NE (150 mg/day) for 4 weeks, followed by surgery and adjuvant erlotinib or vinorelbine plus cisplatin therapy depending on observed NE response. TME changes were assessed based on gene expression analysis and mutation profiling.
Results
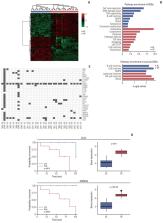
A total of 26 patients were enrolled; the median age was 61, 69% were female, 88% were stage IIIA, and 62% had L858R mutation. Among 25 patients who received NE, the objective response rate was 72% (95% confidence interval [CI], 52.4 to 85.7). The median disease-free and overall survival (OS) were 17.9 (95% CI, 10.5 to 25.4) and 84.7 months (95% CI, 49.7 to 119.8), respectively. Gene set enrichment analysis in resected tissues revealed upregulation of interleukin, complement, cytokine, transforming growth factor β, and hedgehog pathways. Patients with upregulated pathogen defense, interleukins, and T-cell function pathways at baseline exhibited partial response to NE and longer OS. Patients with upregulated cell cycle pathways at baseline exhibited stable/progressive disease after NE and shorter OS.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.
- Record: found
- Abstract: found
- Article: not found