- Record: found
- Abstract: found
- Article: found
Differential gene expression in recombinant Pichia pastoris analysed by heterologous DNA microarray hybridisation
Read this article at
Abstract
Background
Pichia pastoris is a well established yeast host for heterologous protein expression, however, the physiological and genetic information about this yeast remains scanty. The lack of a published genome sequence renders DNA arrays unavailable, thereby hampering more global investigations of P. pastoris from the beginning. Here, we examine the suitability of Saccharomyces cerevisiae DNA microarrays for heterologous hybridisation with P. pastoris cDNA.
Results
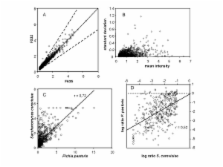
We could show that it is possible to obtain new and valuable information about transcriptomic regulation in P. pastoris by probing S. cerevisiae DNA microarrays. The number of positive signals was about 66 % as compared to homologous S. cerevisiae hybridisation, and both the signal intensities and gene regulations correlated with high significance between data obtained from P. pastoris and S. cerevisiae samples. The differential gene expression patterns upon shift from glycerol to methanol as carbon source were investigated in more detail. Downregulation of TCA cycle genes and a decrease of genes related to ribonucleotide and ribosome synthesis were among the major effects identified.
Conclusions
We could successfully demonstrate that heterologous microarray hybridisations allow deep insights into the transcriptomic regulation processes of P. pastoris. The observed downregulation of TCA cycle and ribosomal synthesis genes correlates to a significantly lower specific growth rate during the methanol feed phase.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Book: not found
Molecular Cloning : A Laboratory Manual
- Record: found
- Abstract: found
- Article: not found
An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains.
- Record: found
- Abstract: found
- Article: not found