- Record: found
- Abstract: found
- Article: found
Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity
Read this article at
Abstract
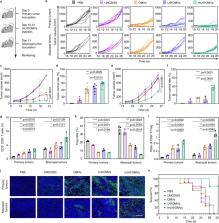
Efferocytosis inhibition is emerging as an attractive strategy for antitumor immune therapy because of the subsequent leak of abundant immunogenic contents. However, the practical efficacy is seriously impeded by the immunosuppressive tumor microenvironments. Here, we construct a versatile nanosystem that can not only inhibit the efferocytosis but also boost the following antitumor immunity. MerTK inhibitor UNC2025 is loaded into the bacterial outer membrane vesicles (OMVs), which are then modified with maleimide (mU@OMVs). The prepared mU@OMVs effectively inhibits the efferocytosis by promoting the uptake while preventing the MerTK phosphorylation of tumor associated macrophages, and then captures the released antigens through forming universal thioether bonds. The obtained in situ vaccine effectively transfers to lymph nodes by virtue of the intrinsic features of OMVs, and then provokes intense immune responses that can efficiently prevent the growth, metastasis and recurrence of tumors in mice, providing a generalizable strategy for cancer immunotherapy.
Abstract
Efferocytosis inhibition leads to the release of immunogenic contents into the tumor microenvironment. Here the authors developed a nanosystem that inhibits MerTK-mediated efferocytosis and captures tumor-associated agents to enhance antitumour immunity.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Release of chromatin protein HMGB1 by necrotic cells triggers inflammation.
- Record: found
- Abstract: found
- Article: not found