- Record: found
- Abstract: found
- Article: found
Bone Marrow CD133 + Stem Cells Ameliorate Visual Dysfunction in Streptozotocin-induced Diabetic Mice with Early Diabetic Retinopathy
Read this article at
Abstract
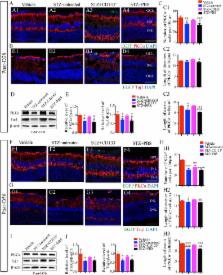
Diabetic retinopathy (DR), one of the leading causes of vision loss worldwide, is characterized by neurovascular disorders. Emerging evidence has demonstrated retinal neurodegeneration in the early pathogenesis of DR, and no treatment has been developed to prevent the early neurodegenerative changes that precede detectable microvascular disorders. Bone marrow CD133 + stem cells with revascularization properties exhibit neuroregenerative potential. However, whether CD133 + cells can ameliorate the neurodegeneration at the early stage of DR remains unclear. In this study, mouse bone marrow CD133 + stem cells were immunomagnetically isolated and analyzed for the phenotypic characteristics, capacity for neural differentiation, and gene expression of neurotrophic factors. After being labeled with enhanced green fluorescent protein, CD133 + cells were intravitreally transplanted into streptozotocin (STZ)-induced diabetic mice to assess the outcomes of visual function and retina structure and the mechanism underlying the therapeutic effect. We found that CD133 + cells co-expressed typical hematopoietic/endothelial stem/progenitor phenotypes, could differentiate to neural lineage cells, and expressed genes of robust neurotrophic factors in vitro. Functional analysis demonstrated that the transplantation of CD133 + cells prevented visual dysfunction for 56 days. Histological analysis confirmed such a functional improvement and showed that transplanted CD133 + cells survived, migrated into the inner retina (IR) over time and preserved IR degeneration, including retina ganglion cells (RGCs) and rod-on bipolar cells. In addition, a subset of transplanted CD133 + cells in the ganglion cell layer differentiated to express RGC markers in STZ-induced diabetic retina. Moreover, transplanted CD133 + cells expressed brain-derived neurotrophic factors (BDNFs) in vivo and increased the BDNF level in STZ-induced diabetic retina to support the survival of retinal cells. Based on these findings, we suggest that transplantation of bone marrow CD133 + stem cells represents a novel approach to ameliorate visual dysfunction and the underlying IR neurodegeneration at the early stage of DR.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Diabetes impairs hematopoietic stem cell mobilization by altering niche function.
- Record: found
- Abstract: found
- Article: not found
