- Record: found
- Abstract: not found
- Article: not found
Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants
letter
Jingyou Yu , Ph.D.,
Ai-ris Y. Collier , M.D.,
Marjorie Rowe , B.S.,
Fatima Mardas , B.S.,
John D. Ventura , Ph.D.,
Huahua Wan , M.S.,
Jessica Miller , B.S.,
Olivia Powers , B.S.,
Benjamin Chung , B.S.,
Mazuba Siamatu , B.A.,
Nicole P. Hachmann , B.S.,
Nehalee Surve , M.S.,
Felix Nampanya , B.S.,
Abishek Chandrashekar , M.S.,
Dan H. Barouch , M.D., Ph.D.
16 March 2022
Keyword part (code): 18Keyword part (keyword): Infectious DiseaseKeyword part (code): 18_2Keyword part (keyword): VaccinesKeyword part (code): 18_6Keyword part (keyword): Viral InfectionsKeyword part (code): 18_12Keyword part (keyword): Coronavirus ,
18,
Infectious Disease,
Keyword part (code): 18_2Keyword part (keyword): VaccinesKeyword part (code): 18_6Keyword part (keyword): Viral InfectionsKeyword part (code): 18_12Keyword part (keyword): Coronavirus ,
18_2,
Vaccines,
18_6,
Viral Infections,
18_12,
Coronavirus
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
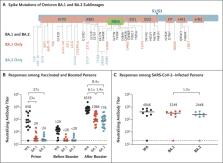
To the Editor: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529
(omicron) variant has three major sublineages: BA.1, BA.2, and BA.3.
1
BA.1 rapidly became dominant and has shown substantial escape from neutralizing antibodies
induced by vaccination.
2-4
The number of cases of BA.2 has recently increased in many regions of the world, suggesting
that BA.2 has a selective advantage over BA.1. BA.1 and BA.2 share multiple common
mutations, but each also has unique mutations
1
(Figure 1A). The ability of BA.2 to evade neutralizing antibodies induced by vaccination
or infection is unclear.
We evaluated neutralizing antibody responses against the parental WA1/2020 strain
of the virus, as well as against the omicron BA.1 and BA.2 variants, in 24 persons
who had been vaccinated and boosted with the BNT162b2 mRNA vaccine (Pfizer–BioNTech)
5
and had not had infection with SARS-CoV-2 and in 8 persons with a history of SARS-CoV-2
infection, irrespective of vaccination status. Demographic and clinical characteristics
of the study population are provided in Tables S1 and S2 in the Supplementary Appendix,
available with the full text of this letter at NEJM.org.
After the initial two doses of the BNT162b2 vaccine, the median pseudovirus neutralizing
antibody titers against WA1/2020, BA.1, and BA.2 were 658, 29, and 24, respectively
(Figure 1B), indicating that the median neutralizing antibody titer against WA1/2020
was 23 and 27 times those for BA.1 and BA.2, respectively. Six months after the initial
vaccination, the median neutralizing antibody titers declined to 129 for WA1/2020
and to less than 20 for both BA.1 and BA.2. Two weeks after the third dose (booster)
of the BNT162b2 vaccine, the median neutralizing antibody titers increased substantially
to 6539 for WA1/2020, 1066 for BA.1, and 776 for BA.2, indicating that the median
neutralizing antibody titer against WA1/2020 was 6.1 and 8.4 times those for BA.1
and BA.2, respectively (Figure 1B). The median BA.2 neutralizing antibody titer was
lower than the median BA.1 neutralizing antibody titer by a factor of 1.4.
We next evaluated neutralizing antibody titers in the 8 persons with a history of
SARS-CoV-2 infection (see Tables S1 and S2) at a median of 14 days after SARS-CoV-2
infection, which was diagnosed during a time when the omicron BA.1 sublineage was
responsible for more than 99% of new infections. The median neutralizing antibody
titers were 4046 for WA1/2020, 3249 for BA.1, and 2448 for BA.2 (Figure 1C). The median
BA.1 neutralizing antibody titer was 1.3 times the median BA.2 neutralizing antibody
titer. The one person who did not have detectable neutralizing antibody titers was
unvaccinated, and the serum sample was obtained 4 days after diagnosis of SARS-CoV-2
infection.
Overall, these data show that neutralizing antibody titers against BA.2 were similar
to those against BA.1, with median titers against BA.2 that were lower than those
against BA.1 by a factor of 1.3 to 1.4. A third dose of the BNT162b2 vaccine was needed
for induction of consistent neutralizing antibody titers against either BA.1 or BA.2.
3,4
Moreover, in vaccinated persons who had presumably been infected with BA.1, robust
neutralizing antibody titers against BA.2 developed, which suggests a substantial
degree of cross-reactive natural immunity. These findings have important public health
implications and suggest that the increasing frequency of BA.2 in the context of the
BA.1 surge is probably related to increased transmissibility rather than to enhanced
immunologic escape.
Related collections
Most cited references5
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine

- Record: found
- Abstract: found
- Article: found
Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa
Raquel Viana, Sikhulile Moyo, Daniel G. Amoako … (2022)

- Record: found
- Abstract: found
- Article: found
Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization
Sandile Cele, Laurelle Jackson, David Khoury … (2021)
