- Record: found
- Abstract: found
- Article: found
Genetic Approaches for the Treatment of Facioscapulohumeral Muscular Dystrophy
Read this article at
Abstract
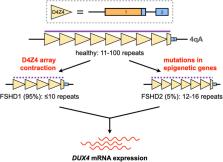
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disorder characterized by progressive, asymmetric muscle weakness at the face, shoulders, and upper limbs, which spreads to the lower body with age. It is the third most common inherited muscular disorder worldwide. Around 20% of patients are wheelchair-bound, and some present with extramuscular manifestations. FSHD is caused by aberrant expression of the double homeobox protein 4 ( DUX4) gene in muscle. DUX4 codes for a transcription factor which, in skeletal muscle, dysregulates numerous signaling activities that culminate in cytotoxicity. Potential treatments for FSHD therefore aim to reduce the expression of DUX4 or the activity of its toxic protein product. In this article, we review how genetic approaches such as those based on oligonucleotide and genome editing technologies have been developed to achieve these goals. We also outline the challenges these therapies are facing on the road to translation, and discuss possible solutions and future directions
Related collections
Most cited references137
- Record: found
- Abstract: found
- Article: not found
A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity.
- Record: found
- Abstract: found
- Article: found
CRISPR–Cas9 Structures and Mechanisms
- Record: found
- Abstract: found
- Article: not found